In silico simulation methods have the potential to revolutionize medical device approval by reliably replacing in vitro and in vivo testing. By moving to the virtual patient, there are numerous advantages for medical device manufacturers: early intervention in product development, safe testing and fitting, avoidance of time-consuming and costly human and animal trials. The innovative Swiss company SDS already benefits from the possibilities of FEM analysis in the approval process.

1. Introduction
Healthcare is a sector in which companies must comply with strict obligations imposed on health and by regulatory organizations. Before bringing medical products to market, companies must meet a series of certifications and testing criteria to ensure the safety and quality of the products for patients.
2. Long and expensive testing procedures
Thanks to material science advancements, the dental industry has recently shown great promise in developing durable dental solutions, such as dental implants.
Nonetheless, a massive amount of time and money is still devoted to the testing procedures to comply with regulations requested by authorities.
There is stiff competition in the dental industry to deliver high-quality, patient-specific, and affordable dental implants in a timely manner.
Designers typically rely heavily on physical experimentation to test and validate their final products. This results in tremendous money and time spent physically manufacturing and testing each implant variation.
3. Objectives
In this article, we present a real-life case study that highlights a unique approach to solving approval challenges within the dental industry: the utilization of finite element method (FEM) analysis. While it is widely acknowledged that FEM analysis can serve as a valuable complement to physical experiments, concerns regarding its practical implementation are emerging.
Let’s dive into this fascinating real-life story to discover how a regulatory agency supported the approval of a dental product using FEM with our Simq platform.
4. Challenge: Implant variations
Swiss Dental Solutions (SDS), our valued customer, is a fast-growing company specializing in groundbreaking dental treatment concepts, particularly in the area of biomedical implant solutions using ceramic implants.
SDS has recently set its sights on introducing a series of ceramic implants to the U.S. market, having satisfactorily completed mechanical testing, as shown in Fig. 1. However, the U.S. Food and Drug Administration (FDA) has requested further evidence to confirm the durability of the ceramic implants worldwide and at higher loads during fatigue testing, specifically for additional variants.
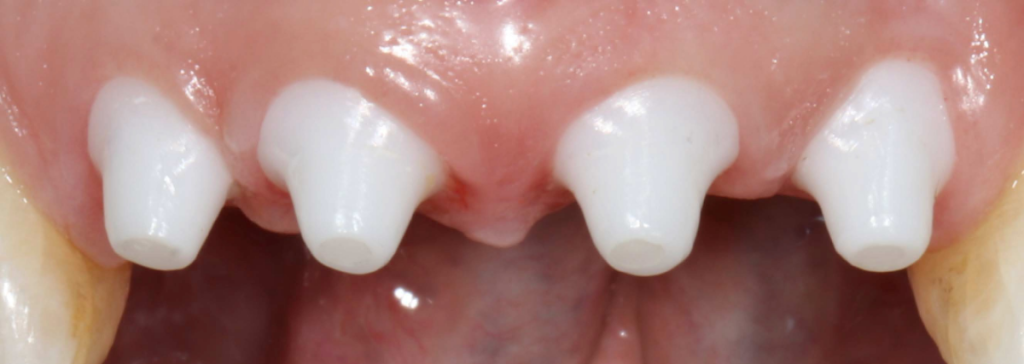
Swiss Dental Solutions had a tight deadline of 120 days to address the FDA’s request, making it impractical to perform physical testing on all implant variants within that time frame. It was crucial for the company to find a solution promptly, as any delays could potentially jeopardize the entire approval process.
Consequently, SDS initiated a search for a viable alternative to experimental testing that could effectively evaluate the performance of variants under higher loads.
5. Solution: Physics-based simulations
Our client conducted a series of experimental studies to analyze the fatigue performance of their innovative zirconium oxide dental implants in accordance with the EN ISO 14801 standard.
This standard provides a comprehensive framework for the dynamic testing of dental implants and their premanufactured prosthetic components.
By adhering to this method, dental implants constantly demand thorough evaluation to ensure they meet stringent quality and safety standards.

“Simq’s FEM analysis allowed us to answer the FDA’s inquiries faster at a critical approval step of our product and minimize risk by reducing costly and time-consuming physical testing.”
Dr. Karl Ulrich Volz, CEO, SDS Swiss Dental Solutions
Utilizing Simq’s FEM analysis tool, SDS conducted numerical simulations on identical implant geometry and embedding components, including the test cylinder and hemispherical loading cap, as depicted in Fig. 2.
The FEA analysis was then compared to previously conducted experimental results to assess the accuracy of the numerical simulations.
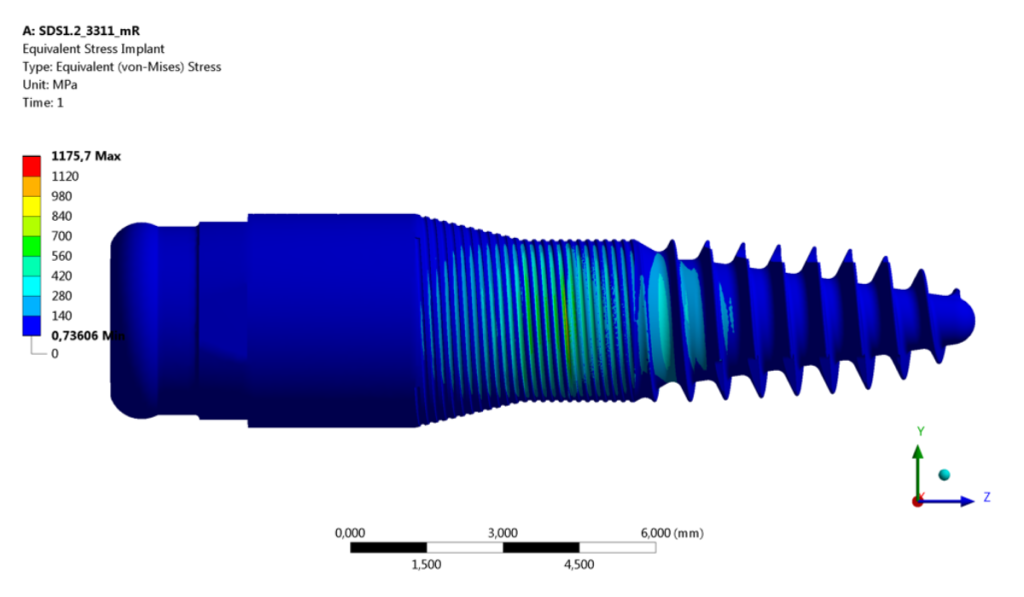
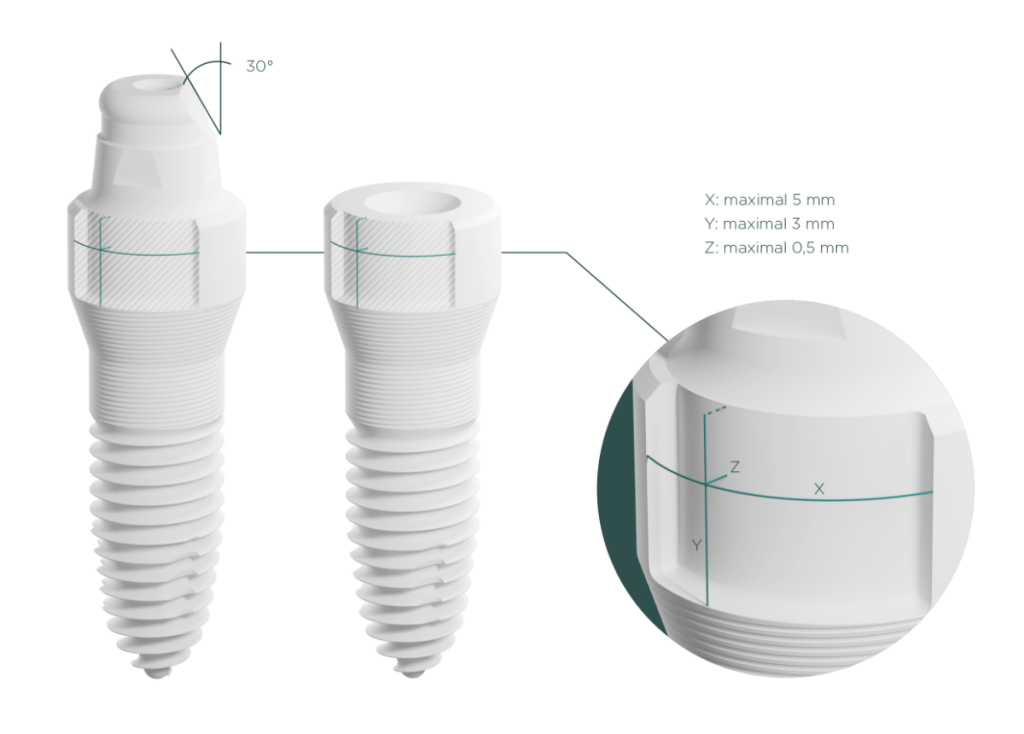
Additionally, SDS took a step further by comparing different variants with varying abutment heights and maximum milled depths of the grindable implants.
The results were carefully analyzed, focusing on parameters that could provoke fatigue damage or cracking of the ceramics, such as equivalent von Mises stress, maximum principal stress, normalized stresses, and other crucial factors.
6. Results
Reliable: Through FEM analysis conducted with Simq, SDS has demonstrated that the stress distribution and maximum stress values of the worst-case variants, which have already undergone physical testing, are not significantly different from the numerically analyzed variants.
Cost-effective: Modifying the post height and performing grinding on the bone implants did not have a significant impact on the stress distribution in these critical areas, as shown in Fig. 3. Thus, thanks to the FEM analysis, SDS confirmed that overall health over the lifetime of the variants remained unaffected.
Timely: As a result, timely additional physical tests could be saved, and the market approval for the US was secured without any delays.
About Simq
Simq is a regulatory platform and certified physics-based simulation software for medical diagnostics. We provide deeper insights into diagnostics and therapeutics and in-depth information for developing state-of-the-art medical devices through innovative technology.
Customers like Medartis can personalize the treatment and diagnosis using patient-specific data instead of empirical data, enabling evidence-based and patient-centered planning and decision-making before the treatment.

Simq is a certified simulation service provider and software manufacturer in the field of medicine and medical technology and is one of the pioneers of in silico medicine.





