Revolutionizing Orthopedics with medartis - Explore Now!

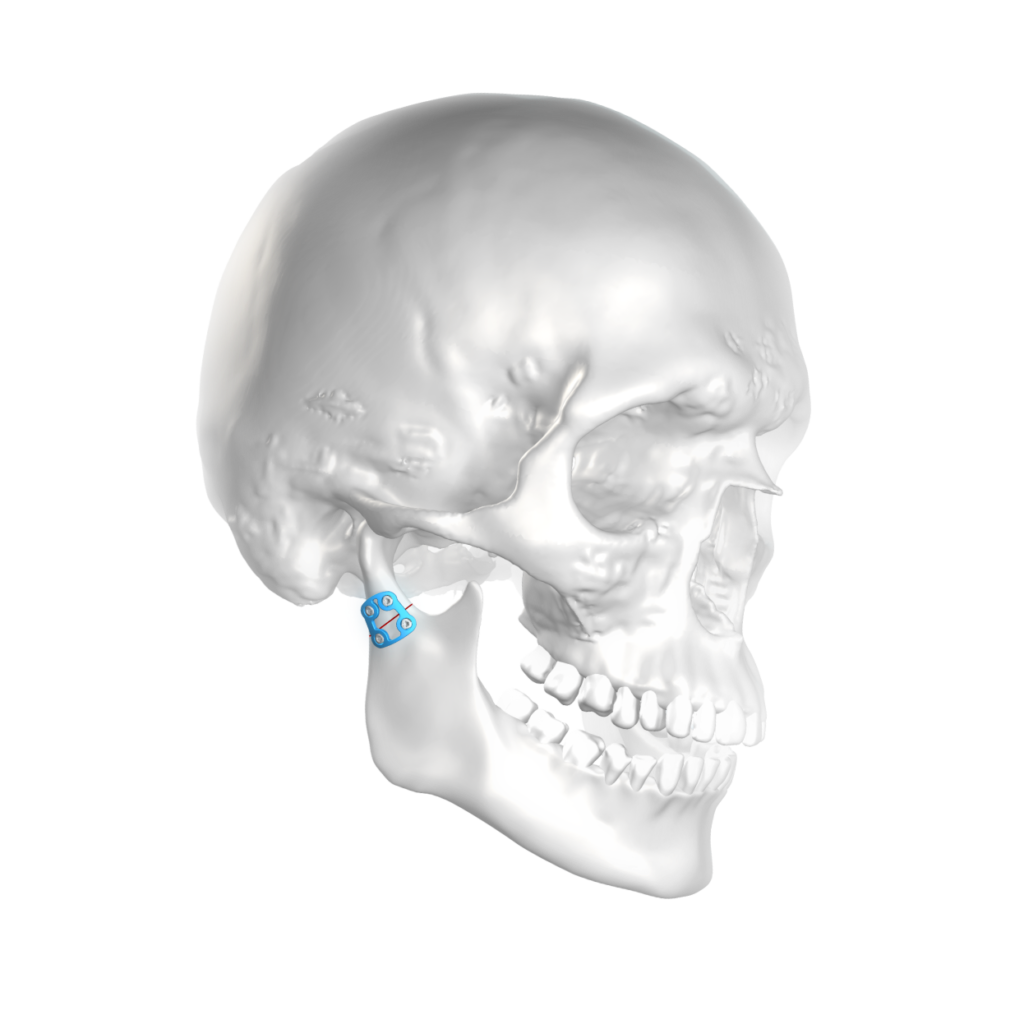
The objective of the analysis is the finite element analysis of different osteosyntheses on the mandible. The clinical results of different implant systems are to be analyzed and compared with each other by means of FE simulations. Thus, selected clinical findings can also be technically substantiated by means of FE analyses.


“With Simq and their software Simq VIT, we were able to prove the performance of our products in a scientific study in cooperation with the University Hospital Marburg and Prof. Neff. The potential of simulation in this field is for us as a manufacturer an important factor and way to secure the quality of our products in the future.”
Adrian Spiegel / Head Research/Testing / Medartis AG

Success Story
Finite element analysis of different osteosyntheses on the mandible
About the customer
As innovation leader, Medartis is advancing technologies and solutions for osteosynthesis in the fields of oral and maxillofacial (OMF) surgery, as well as upper and lower extremities.

The task
For a medical technology manufacturer, the safety of its products is paramount. This also includes their continuous monitoring after they have been placed on the market, as they are only then used practically over an adequate period of time and in a larger number of users or patients. Against this background, we were able to simulatively test an existing product portfolio in patient-specific situations for our customer medartis.

The solution
With the docq VIT (VIT = Virtual Implant Testing) software developed by Simq, physiological load boundary conditions can be applied to the implant very easy and flexible in order to simulate the loading of the products in the body after implantation. In this way, the data previously collected in the post-market clinical follow-up and its observations could be reproduced and substantiated in silico. The results collected in the simulation study could be used to produce a scientific publication and to enhance the technical file. This example impressively demonstrates that simulation can be used to improve the continuous post-market surveillance of medical devices and to objectify and evaluate clinical findings.

The result
For our customer medartis, we used the possibilities of simulation with our docq VIT software for the confirmation of clinical results in cooperation with the University Hospital of Marburg and Prof. Neff. This allowed us to prove the clinical performance and safety of specific ostheosynthesis implants in the context of post market surveillance (PMS) and to identify potential risks in practical use.
Additional sources
Paper by Prof. Andreas Neff
Written title of the paper, can also be a bit longer
3.4 Mb





