Together we make an impact on patients' lives
As a pioneer of in silico medicine, Simq explores and identifies new possibilities to optimize patient care. We drive the adoption of innovative and new technologies and provide development and consulting services to medical device manufacturers.
Our software solutions help healthcare providers, physicians and their patients to get the best diagnosis and therapy.
Trusted by



Trusted by




Why we are here
We believe that every patient deserves the best care possible. We believe that personalized medicine is the future, and we want to make our technology and expertise available to clinicians and manufacturers to enable safer personalized medical products, diagnostics and treatments.


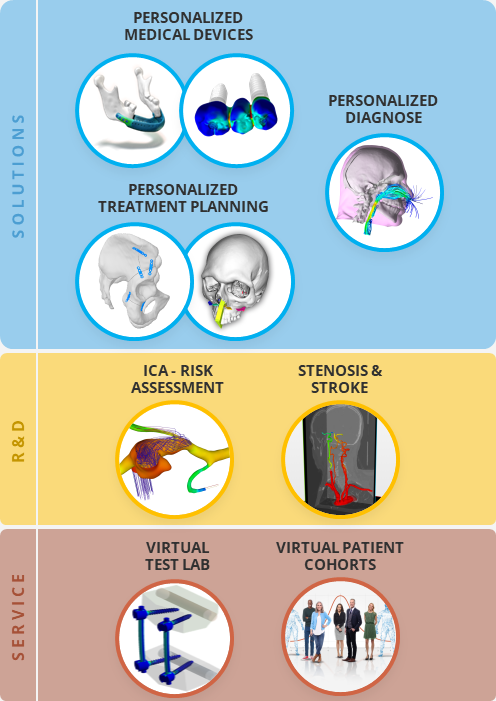
How we deliver
Simq brings biomechanics and in silico technologies from research to clinical application via our cutting-edge technology platform, giving physicians access to more information and personalized insights. To ensure that as many patients as possible benefit from our solutions, we also make the basic technology available to other medical device manufacturers.
What we do
We provide a development platform with a compliant framework to democratize (physics-based) simulation and make it accessible to healthcare professionals. Our team of experts is committed to challenging the status quo and delivering the highest quality to make the potential of digital twin simulation, artificial intelligence and machine learning broadly available.
Medical software based on our

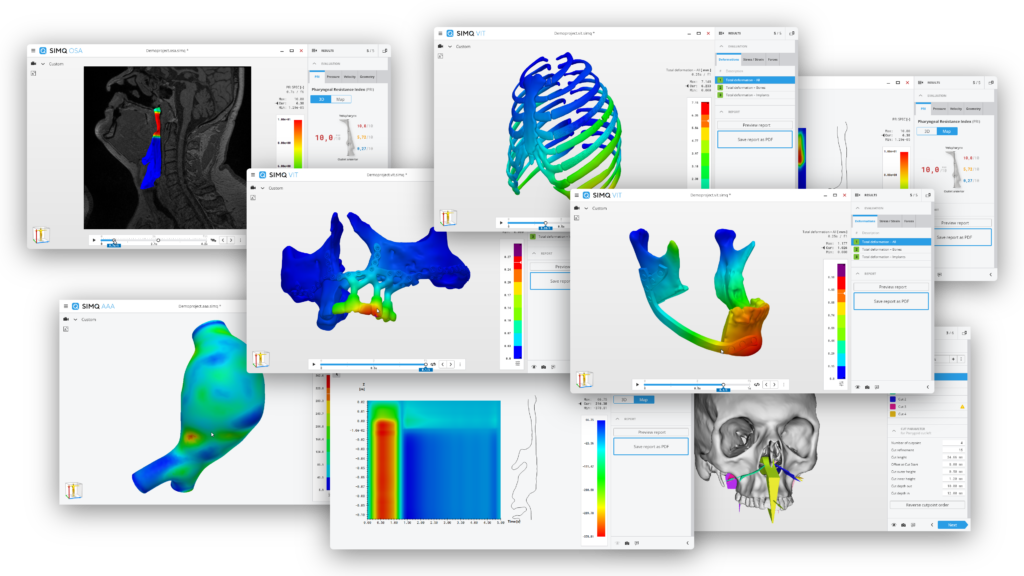
We are Simq.
Our Mission & Vision Statements
Mission: To accelerate the adoption of personalized patient care through simulation.
Vision: Our combination of cutting-edge and established technologies sets new standards in patient care, improves processes and outcomes, and ultimately benefits the entire healthcare system.
March 1, 2015
Corporate Start-Up
The company was founded in 2015 and is part of the CADFEM Group.
April, 2018
ISO 13485 Certified
Since 2018 Simq is a certified software manufacturer for medical devices according to the EU MDR guidelines.
December, 2021
Investment
Simq closed a Series A round with more than €4 M. South Korean CAE specialist TAE SUNG S&E, Inc. invests together with CADFEM International.
June 15, 2022
Rebranding
CADFEM Medical changes the company name to Simq.
Q2, 2024
CE Approval
Simq releases its first medical product Simq OSA.

Simq is a certified simulation service provider and software manufacturer in the field of medicine and medical technology and is one of the pioneers of in silico medicine.

Simq is committed to the standardization and broader application of in silico medicine as part of the Avicenna Alliance, thereby ensuring safe, affordable and cost-effective healthcare.
The team behind Simq

Arkaniva Sarkar
Working student AI/ML

Andrey Shapovalov
Software Developer
Advisory Board
Our partners












































