
Digital verification and compliance with dental workflow regulatory standards is paramount in a rapidly evolving dental technology landscape.
Introducing new regulations for custom-made devices in the EU and the US has reshaped the industry, making it essential for dental professionals to stay ahead of the curve.
This blog introduces the revolutionizing Simq VIT Dental Workflow, designed to virtually test dental restorations and support documentation according to EU MDR, MDCG Q&A 2021-03 and FDA`s Leap Frog Guidance on additive manufacturing.
EU MDR Requirements:
The European Union Medical Device Regulation (MDR) places stringent requirements on medical device manufacturers to ensure the safety and performance of their products.
Some key aspects include:
- Classification: Medical devices are classified into different classes based on their intended use and potential risks. Higher-risk devices are subject to more rigorous conformity assessment procedures.
- Technical Documentation: Manufacturers must create and maintain comprehensive technical documentation demonstrating MDR compliance. This includes design, manufacturing, clinical evaluation, and risk management information.
- Post-Market Surveillance (PMS): Continuous monitoring of devices on the market is required. Manufacturers must collect and analyze data on the performance and safety of their devices and take appropriate corrective actions if issues arise.
- Unique Device Identification (UDI): Devices must bear a UDI, facilitating traceability and post-market surveillance.
- Notified Bodies: Many devices require the involvement of a Notified Body, an independent third-party organization that assesses the conformity of the device with the MDR.
FDA’s “Leap Frog” Guidance on Additive Manufacturing:
As of my last update, the FDA had been working on guiding additive manufacturing (3D printing) of medical devices.
The “Leap Frog” guidance emphasizes technological advancements and innovative approaches to facilitate the development of safe and effective 3D-printed medical products.
Some general points may include:
- Material Characterization: Comprehensive understanding and characterization of the materials used in 3D printing, including considerations for variability in the printing process.
- Quality Systems: Adherence to established quality systems to ensure consistency and reliability in the manufacturing process.
- Biocompatibility: Rigorous testing to demonstrate the biocompatibility of the 3D-printed materials, particularly when the devices come into contact with the patient’s body.
- Post-Processing: Considerations for post-processing steps and their impact on the final product’s performance and safety.
- Design and Manufacturing Controls: Robust design controls and manufacturing processes to ensure the reproducibility and consistency of 3D-printed medical devices.
It’s crucial to refer to the regulatory bodies’ specific guidance documents and updates for the most accurate and current information.
Regulatory and legal requirements are subject to change, and staying informed about the latest guidelines is essential for compliance.
What is Simq VIT Dental Workflow?
Simq VIT Dental Workflow is a comprehensive solution tailored to address the challenges posed by new regulations.
Our software is designed to make the digital verification of patient-specific implants (PSIs) easy and cybersecure. Our software allows you to design better PSIs in less time by virtually applying physiological or standardized loads to a patient-specific situation.
This helps to reduce liability by providing objective criteria to the clinician and designer team during the decision-making process. To ensure implant safety and efficacy, you can set up highly complex biomechanical simulations within minutes.
Our software also helps you meet new regulatory requirements through virtual testing and automated report generation following FDA guidelines. Our simulation business models have been verified and validated by academia and industry leaders.
It empowers dental professionals to verify the mechanical strength and performance of both the traditional dental bridge, and implant-supported dental bridges themselves.
Key Features of Simq VIT Dental Workflow
Simq VIT Dental Workflow introduces an innovative approach to physical testing, transforming the conventional methods of benchtop assessments.
The key features of this system include the recalibration of physical benchtop tests, taking into account known stability and damage behavior.
This ensures a more accurate and reliable evaluation of materials and structures through digital verification.
Moreover, Simq VIT Dental Workflow incorporates a thorough assessment of failure criteria and safety factors, ensuring robust evaluations.
By redefining the standards of physical testing in dental workflows, this technology sets a new benchmark for precision and efficiency in the dental industry.
Simq VIT Dental Workflow goes beyond conventional practices by meticulously comparing benchtop results with patient-specific scenarios, ensuring that the testing environment closely mirrors real-world conditions.
As the technology validates its strength with well-defined safety factors, this approach provides another level of precision in dental workflows.
The proof of strength not only enhances the reliability of the results but also instills confidence in the applicability of these findings to diverse clinical situations.
This commitment to precision in the comparison process distinguishes Simq VIT Dental Workflow as an indispensable tool for dental professionals. Most dentists seek the highest standards of accuracy and relevance in their assessments of dental practice.
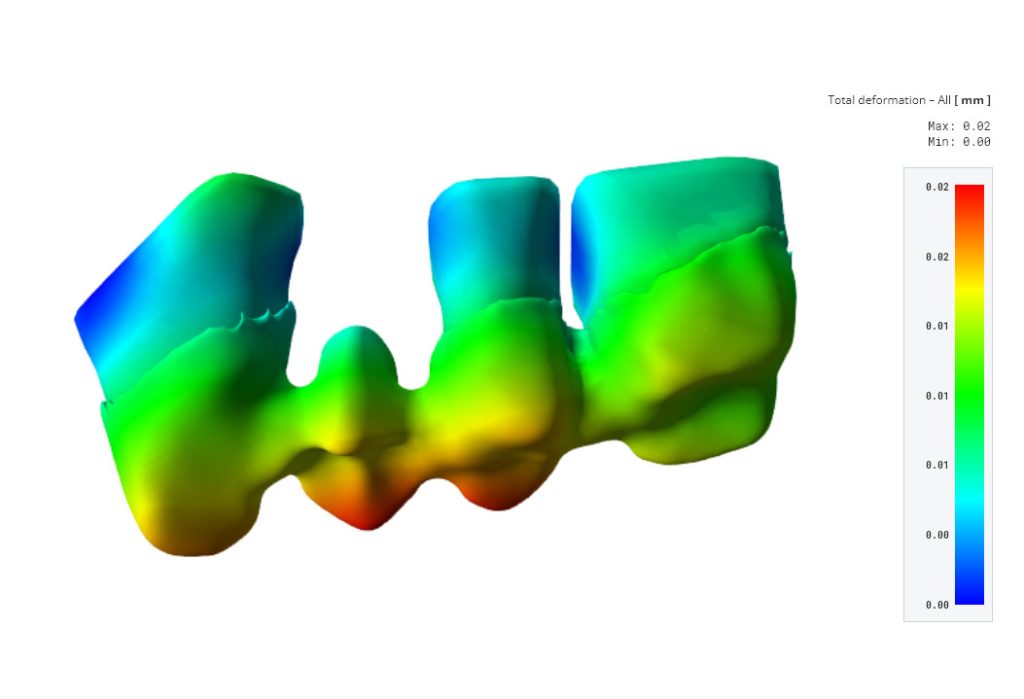
Benefits for Dental Professionals
Simq VIT Dental Workflow offers compelling business benefits for dental professionals, foremost among them being the assurance of patient-specific tooth safety and stability in tooth restorations.
By employing Simq VIT, dental practitioners can confidently deliver personalized solutions that align with each patient’s unique needs, ensuring a safe and stable outcome through digital verification.
The technology further enhances communication efficiency between dentists’ offices and dental laboratories, streamlining the workflow and promoting a seamless exchange of information.
Additionally, the implementation of Simq VIT contributes to a notable reduction in liability for dentists and dental technicians.
This is achieved by improving implant durability, mitigating potential risks, and reinforcing the overall reliability of dental procedures.
As a result, Simq VIT emerges as a transformative tool in dental practice, elevating the quality of patient care and positively impacting the operational aspects of digital workflow and risk management for all dental care professionals.
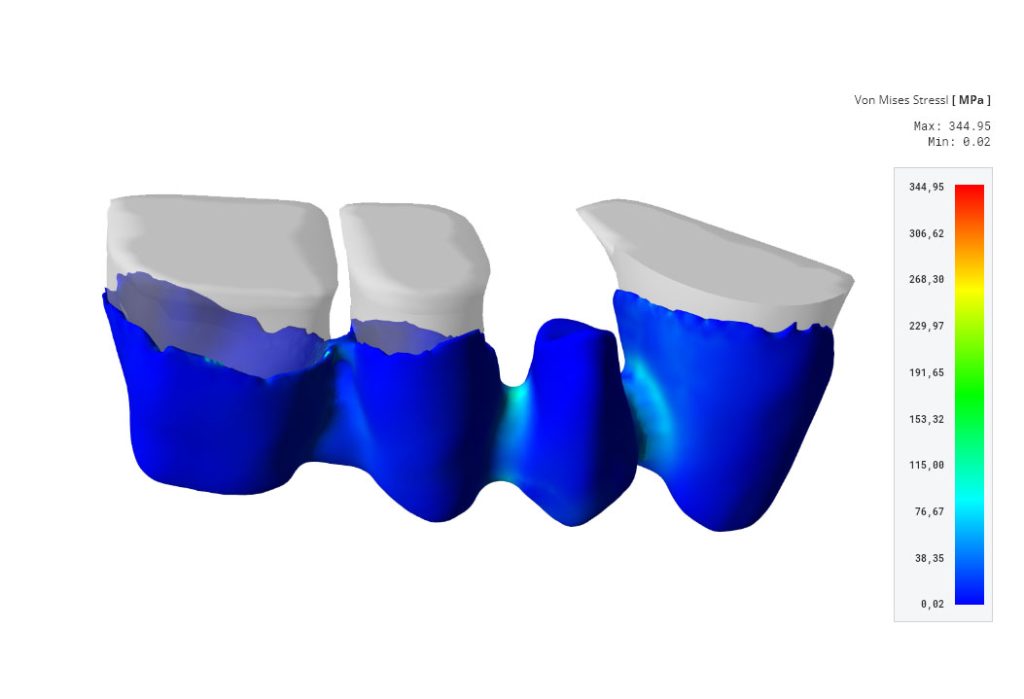
Benefits for Manufacturers
Simq VIT Dental Workflow benefits dental professionals and gives manufacturers a competitive edge and operational and business advantages through digital verification.
Manufacturers can offer extended warranties without compromising profitability, developing a significant market advantage.
This improved warranty offering sets manufacturers apart from competitors, establishing a reputation for reliability and confidence in their products.
The efficiency of Simq VIT extends to customer complaint handling, enabling better tracking and resolution of issues related to implant failure.
This, in turn, reduces the overall number of complaints and enhances customer satisfaction.
Additionally, the system optimizes material handling, ensuring manufacturers achieve optimal efficiency in the production process, ultimately contributing to product quality enhancement.
In conclusion, in response to dynamic regulatory changes in the dental industry, the Simq VIT Dental Workflow stands out from other teeth, as a transformational solution for digital dentistry, providing a streamlined, compliant, and innovative approach to dental implants and restorations.





