Use of in-silico simulation in the FDA approval process for Swiss Dental Solutions
In silico simulation methods have the potential to revolutionize medical device approval by reliably replacing in vitro and in vivo testing.
By moving to the virtual patient, there are numerous advantages for medical device manufacturers: early intervention in product development, safe testing and fitting, and avoidance of time-consuming and costly human and animal trials.
The innovative Swiss company SDS already benefits from the possibilities of FEM analysis in the approval process.

“Simq’s FEM analysis allowed us to quickly answer the FDA’s inquiries at a critical approval step for our product and ensure a smooth product launch in the U.S.”
About the customer
Founded by ceramic pioneer and implantologist Dr. Ulrich Volz, Swiss Dental Solutions offers a comprehensive treatment concept for biomedical implant solutions with ceramic implants.

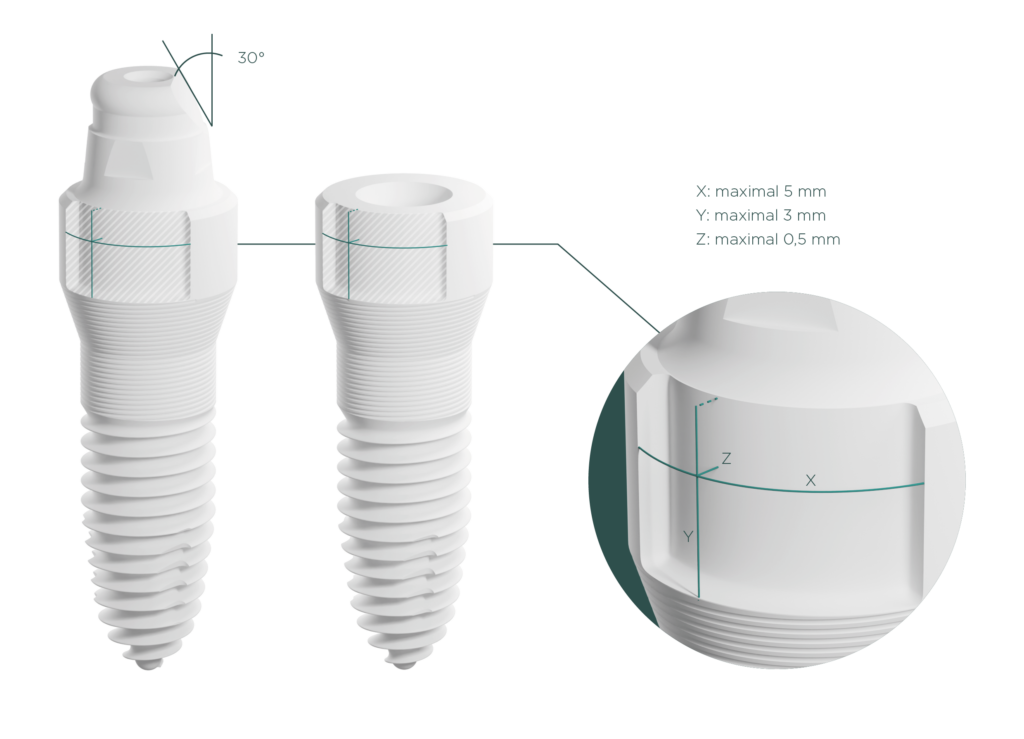
The task
Response to an FDA request: The U.S. Food and Drug Administration (FDA) requested proof that further variants of the ceramic implants already successfully tested by SDS – Swiss Dental Solution in a physical fatigue test do not exhibit higher loads.
The response to this FDA request had to be provided within 120 days.
A renewed physical testing of all variants would not have been possible within the specified timeframe, and thus, the previous efforts to obtain approval for the products in the USA would have been null and void.

The solution
A comparative analysis could be performed through the finite element analysis of the fatigue behavior according to EN ISO 14801 of the variants and the already successfully physically allowed zirconium oxide implants.
The test setup already carried out is replicated in the FEA with the same implant geometry and the same embedding parts (test cylinder and hemispherical loading cap).
Subsequently, the variants with different abutment heights and the maximum recesses of the grindable implants were compared.
The effects of the change can be evaluated based on the calculated quantities, such as the equivalent stress (von Mises), the maximum principal stress, the normal stress in the Z-direction (the bending direction), and others.

The result
The stress distribution and the magnitude of the maximum values are lower and not significantly different from the worst-case variants already physically tested.
Changing the post height as well as grinding the implants did not significantly affect the stress distribution in the critical areas and therefore does not lead to a reduced lifetime of the variants.
Simq’s FEA analyses proved that the already physically tested variants represent the worst-case variants.
The request was successfully answered and thus ensured an undelayed market approval for the US dental implants market.
Additional sources
Paper by Prof. Dr. Dr. Max Mustemann zu Mühlhausen
Written title of the paper, can also be a little longer





