Innovative prosthetic solutions from Simq for KLS Martin

Learn how Simq is helping KLS Martin Group to provide customized and functionally stable immediate rehabilitation to patients with insufficient bone and soft tissue.


“At KLS Martin, we have dealt with the potential and possibilities of simulation in relation to our products at an early stage. In the meantime, simulation has become the standard for supporting the creation and maintenance of technical documentation for our products. It saves us a lot of time and money compared to conventional methods such as test bench, animal, ex-vivo or human testing.”
Frank Reinauer / Head of Innovations and Production Biomaterials / KLS Martin Group

About the customer
KLS Martin Group – “Surgical Innovation is our Passion”
The KLS Martin Group is an internationally active group of companies for innovative medical technology in almost all areas of surgery. With their innovative medical technology solutions such as implant systems, high-frequency surgical devices, surgical lasers, sterilization containers, surgical lights, surgical instruments as well as individual OR solutions, they have set new standards many times.

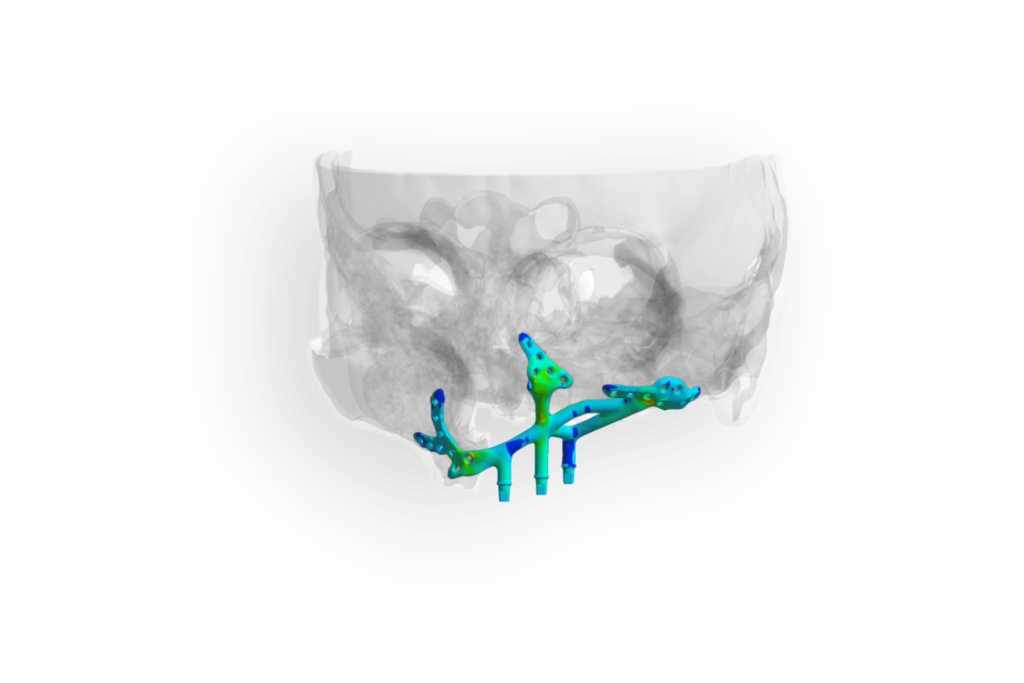
The task
Solid and functional teeth mean quality of life. When teeth are lost, there are various ways to replace them. However, conventional treatment methods are often no options, especially for patients with severe malformations, defects after tumors or accidents.
In order to nevertheless create fixed dentures, a functionally stable one-piece framework is implanted into which the denture is placed. To exclude complications regarding stability, anchoring and strength and to obtain proof of the durability of the patient-specific implant, the situation in the patient must be checked under biomechanical load conditions.

The solution
In order to be able to offer the affected patients a quick and safe solution for fixed dentures, a segmentation from CT data and a simulation of the individual patient situation was first performed postoperatively to calculate the forces and loads occurring in the maxilla. The damage behavior, failure criteria and safety factors are evaluated according to the FKM guideline. In addition, a comparative check of the strength of a patient-specific implant with a physically tested variant is performed.

The result
The products can be demonstrably improved by biomechanical simulation of the implants’ behavior – to the benefit of the patient. The practitioner gets an insight into the functionality of the implants in the functionally stable situation before the intervention and can supplement his technical file (device history file) with objective results from the comparative simulation study. The recommended actions from the simulation allow the implant to be inserted in such a way that inflammation and a possible implant loss resulting from this (stress shielding) can be ruled out.
In addition, the practitioner receives proof of the functionally stable anchoring and sufficient strength of the highly stressed individual implant in the patient.
The patient can thus look forward to a tested, durable implant and fixed teeth after just six weeks.
Additional sources
Paper by Prof. Dr. Dr. Max Mustemann zu Mühlhausen
Written title of the paper, can also be a little longer





